Microbial stoichiometry
In the microbial aspect, stoichiometry defines the quantitative relationship between substrates and product of microbial processes, including biomass formation.

Figure 1: The black box model analyzes the inputs and outputs of the system instead of the processes inside the system itself. All the chemical reactions that occur within the cells are neglected, and the whole fermentation process is viewed as one single reaction, where substrates are converted to biomass and products.

Yield coefficient and exponential growth
In exponential growth, there is a linear relationship between substrate consumption and product formation. The yield coefficient will thereby be constant, which can be calculated as the slope in a plot of the product versus substrate.

Figure 2: During the fermentation, substrate is consumed and biomass and a product are formed. Plotting biomass over substrate shows a linear curve, with Ysx as the slope.
Calculating the yield coefficient
In general, only the most important substrates and products are regarded. For example, for yeast cells, the general stoichiometric equation would be as follows:

The coefficient Y in front of every compound is the yield coefficients. That is the ratio between the change in compound j and the change compound i.

Carbon balance and cmol
For carbon balance (or any elemental balance in the fermentation for that matter), the general stoichiometric equation is used. All the compounds that contain carbon are taken into account:
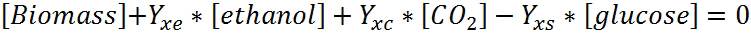
Because only the elemental contribution of biomass and not the specific molecular formula is known, the units are changed to cmol. Relative to cmol, the general composition in the microorganism is CH1.8O0.5N0.2. When converting to cmol, the molecule and the molecular weight are normalized to the carbon content. For example, glucose(C6H12O6) having the molecular weight of 180 g/mol in cmol is CH2O, and it has the molecular weight of 30 g/cmol (simply the original molecular weight divided by the number of carbon atoms). The yields coeffcients can be converted from g/g to cmol/cmol.

After converting the units to cmol, the carbon balance is as follows:
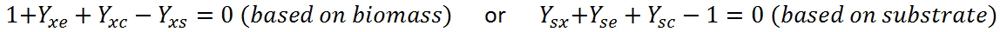
The carbon balance is a good method of controlling the fermented microorganisms. If the carbon balance does not close, there is a lot of unaccounted carbon, which means unknown byproducts are being produced.
Molecular weights
The following elemental molecular weights are useful when calculating the molecular weights of the organic substances:
Mw (Hydrogen) = 1 g/mol
Mw (Carbon) = 12 g/mol
Mw (Nitrogen) = 14 g/mol
Mw (Oxygen) = 16 g/mol
Abbreviation
x: concentration of biomass produced
DW: dry weight, a measurement of biomass
Y: yield factor
SR: initial substrate concentration
s: residual substrate concentration
p: concentration of product
qp: specific rate of product formation (mg product g-1 biomass h -1)
Yxp: yield of product in terms of biomass (g product g-1 biomass)