Preparing Buffers
You can prepare buffers in many different ways. The main method is to mix an acid and its conjugate base, or vice versa, other methods include:
-
Adding a solution of strong base of concentration [Strong base]initial to a solution of weak acid of concentration [HA]initial. - As seen in figure 1a.
-
Adding a solution of strong acid of concentration [Strong acid]initial to a solution of weak base of concentration [B]initial. - As seen in figure 1b.
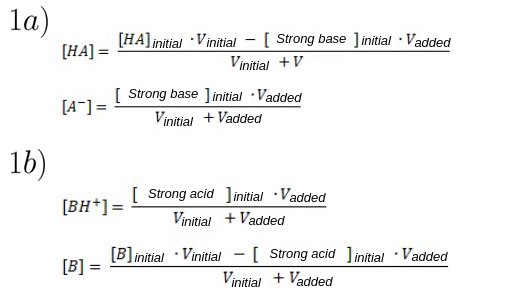
Figure 1: Buffer preparation equations.
1a) Adding a solution of strong base to a solution of weak acid.
1b) Adding a solution of strong acid to a solution of weak base.