Simple Distillation
A simple distillation is defined as a distillation in which the liquid is boiled at one location and all of the resulting gases are immediately condensed and collected in a separate flask. The experimental set-up for a simple distillation is shown below.
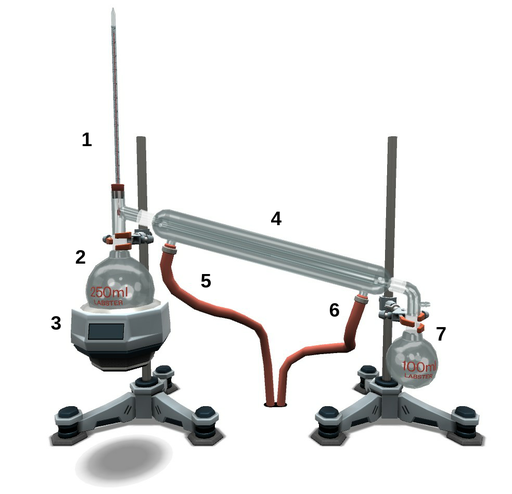
Figure 1. Distillation apparatus. 1. Thermometer just below the sidearm; 2. Distillation flask; 3. Heating mantle; 4. Condenser; 5. Cooling water out; 6. Cooling water in; 7. Collecting flask.
Simple distillations, by definition, are said to have one theoretical plate. A theoretical plate is one vaporization-condensation cycle. What happens in a simple distillation is the mixture is heated to reflux, the vapors travel up the glassware until they hit the cold reflux condenser, the vapors re-condense and are collected in a collection flask – one vaporization-condensation cycle. Simple distillation is only an effective technique to purify a mixture of liquids if the liquids have very different boiling points (typically a difference of >70 oC).